Cardiopulmonary Risk of Waterpipe Smoke: A Meta-Analysis
Matthew Randolph Marshall1*, Marya Ghazipura2, Tanzib Hossain3, Terry Gordon1, Lung-Chi Chen1
1New York University School of Medicine, Department of Environmental Health Sciences, New York University Langone Medical Center, New York, NY
2New York University School of Medicine, Department of Population Health, New York, NY
3New York University School of Medicine Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, New York, NY
Abstract
Purpose: This study aimed to analyze and summarize the effects that waterpipe (WP) smoke has on the cardiopulmonary system through a systematic review and meta-analysis.
Methods: We searched MEDLINE, Embase, Wiley Cochrane Library, Centre for Reviews and Dissemination, CINAHL Plus, and grey literature in March 2017. Our inclusion criteria for the studies were a comparison of WP smokers before and after waterpipe smoking (WPS) or to non-smokers.
Results: Using a random effects meta-analysis, a WPS session was associated with an elevation in systolic blood pressure (SBP) by 6.45 mmHg (95% CI 3.87 to 9.04; p < 0.0001), diastolic blood pressure (DBP) by 3.71 mmHg (95% CI 2.34 to 5.08; p < 0.0001), mean arterial pressure by 5.54 mmHg (95% CI 3.33 to 7.76; p < 0.0001), heart rate by 7.03 bpm (95% CI 4.60 to 9.46; p < 0.0001), carboxyl hemoglobin (COHb) by 4.11% (95% CI 3.38 to 4.84; p < 0.0001), and expired carbon monoxide (CO) by 22.53 ppm (95% CI 15.99 to 29.08; p < 0.0001).
Conclusion: WPS exposure is associated with significant acute increases in cardiopulmonary hemodynamic parameters, along with COHb and expired CO. These findings parallel the acute effects seen with cigarette smoking.
Introduction
According to the 2010 Global Burden of Disease Study, cardiopulmonary diseases are responsible for the top 5 causes of death worldwide1. Within the United States (U.S), mortality data from 2015 shows cardiovascular disease (CVD) ranked as the leading cause of death in the U.S.,2 with about 80% of CVD being preventable,3 and chronic lower respiratory diseases ranked third2. Additionally, within the second leading cause of death in the U.S., cancer, lung and bronchus cancer is the leading cause of mortality in both men and women4. One of the greatest risk factors for developing cardiopulmonary disease is cigarette smoking,5 but with major public health efforts since 1965, the prevalence of cigarette smoking has been declining6.
In contrast to cigarette use, in the last decade, waterpipe (WP) use, commonly referred to as hookah, nargile, shisha, or hubble-bubble, has risen in popularity in Western countries, especially among adolescent populations7-9. In the United States, 8% of adolescents 13 -17 years old and 20% of high-school seniors reported using a WP, according to the 2011 National Youth Tobacco Survey9. The increase in popularity is partially attributable to the misconception that waterpipe smoke (WPS) is less deleterious than cigarette smoke8,10. Nonetheless, similar to cigarettes, an association between WPS and cardiopulmonary disease has been documented11.
Studies have shown that during a typical WPS session, which varies in length from 30 minutes to 90 minutes with the average duration around 45 minutes, there can be acute increases in hemodynamic parameters of cardiopulmonary function such as systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), and heart rate (HR), as well as an increase in expired carbon monoxide (CO) and carboxyl hemoglobin (COHb)7,12,13.
Although these common parameters of cardiopulmonary function appear to correlate with WPS in some studies, the short-term and long-term health effects of WPS on the cardiopulmonary system are still not well understood. Thus, this meta-analysis aims to summarize the effects of WPS on several common hemodynamic parameters of cardiopulmonary function and respiratory markers of tobacco exposure.
Methods
A comprehensive literature review was conducted in March 2017 for all relevant publications up to that date in English using Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid Embase, the Centre for Reviews and Dissemination database Cumulative Index to Nursing & Allied Health Literature (CINAHL Plus), Google Scholar, and the Wiley Cochrane Library. PubMed Reminer was used to help refine the search strategy and ensure all appropriate and relevant terms were included. Medical Subject Heading (MeSH) terms and keywords that were included are detailed in Table 1. A grey literature search was also conducted by hand searching OAIster, Open Grey, Grey Literature Report, and Proceedings of the National Academy of Sciences. All studies comparing the before and after hemodynamic effects of WPS were included, including several that analyzed smokers who used both waterpipes and cigarettes. Studies that did not distinguish between tobacco use or indicate a proper cessation from cigarette smoke were excluded. The references for selected studies were hand searched to identify any additional literature. The process of selecting studies for the final inclusion in this systematic review, as well as the reasons for exclusion, is outlined in Figure 1. Relevant data were extracted on study design and setting, population, the type of WP being used, and outcomes of HR, SBP, DBP, expired CO, and COHb. Two authors independently assessed the relevance and quality of all included studies. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria was used to ensure thorough and transparent evaluation of all included literature14.
Table 1. MeSH Headings Used to Identify Relevant Studies
| Exposure |
| 1. “Hookah pipe smoking” |
| 2. “Waterpipe smoking” |
| 3. “Water-pipe smoking” |
| 4. “Shisha smoking” |
| 5. “Shisha” |
| 6. “Nargile” |
| 7. “Goza” |
| 8. “Hubble bubble” |
| 9. “1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8” |
| Outcome |
| 10. “Cardiovascular disease” |
| 11. “Respiratory system” |
| 12. “Blood pressure” |
| 13. “Respiratory” |
| 14. “Baroeflex” |
| 15. “Blood pressure” |
| 16. “Cardiorespiratory” |
| 17. “Cardiopulmonary” |
| 18. “Heart rate” |
| 19. “10 Or 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18” |
| Combined Terms |
| 20. “9 AND 19” |
Abbreviations: MeSH, Medical Subject Headings
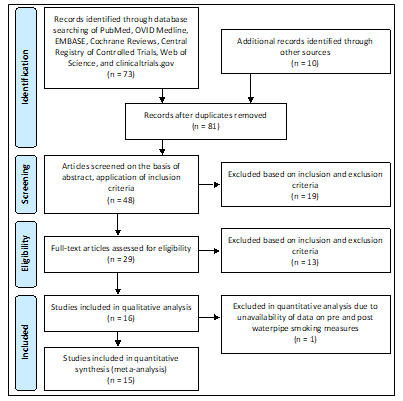
Figure 1. Process of Inclusion of Studies
Abbreviations: n, sample size
The quality of the body of evidence for each hemodynamic parameter and respiratory marker of tobacco exposure was assessed in accordance to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group15. Using this tool, outcomes from each study were graded as high, moderate, low, or very low quality, based on the methodology reported in the individual studies. Risk of bias, inconsistency, indirectness, imprecision, and publication bias were taken into account, and any limitations in these fields resulted in downgrading the quality of evidence.
Using the “meta” and “forestplot” packages in R, meta-analyses were conducted to derive effect estimates of all relevant cardiopulmonary health markers16,17. Mean differences with corresponding 95% confidence intervals were calculated using a random effects model to account for the high heterogeneity (I2 statistic) and corresponding forest plots were created to graphically depict the results.
Results
As noted in Figure 1, the literature search yielded a total of 73 studies from the databases searched, and another 10 studies were identified through other sources. After the removal of duplicates, 81 studies were screened for titles, and 48 studies remained for abstract screening and application of the inclusion criteria. Of these abstracts, 29 studies were eligible for full-text review, and 15 studies were ultimately included in the meta-analysis altogether. The inter-rater reliability was computed using the Cohen’s kappa coefficient and was calculated to be 0.91. The results of the synthesis are summarized in Table 2.
Table 2. Markers of Cardiopulmonary Function in Waterpipe Smoking
| Author, Year | Study Design and Location | Sample Size and Characteristics | Waterpipe & Equipment Properties | Comments and Limitations |
|---|---|---|---|---|
| Shafagoj et al. 200212 | • Cross-sectional design • Volunteers smoked for 45-minutes in a well ventilated room • Tobacco cessation prior to beginning of study: 84 hours • School of Medicine in Amman, Jordan |
• Age range: 20 – 45 years old • Average age in years (Mean ± SD): 27 ± 8 • Average WP use in years (Mean ± SD): 3.8 ± 2.3 (smoked at least 3 times per week) • n = 18 (100% male) • Convenience sampling |
• WP: Large-size Arghileh • 20 grams of HB Mu'asel • BP measured by mercury sphygmomanometer • CO measured by a trained person using a portable Bedfont EC50-MICRO CO monitor smokerlyser. • HR measurement technique not reported |
• Small sample size • Measured maximum CO after first 5 minutes of smoking, which might not be an indicator of smoke inhaled |
| Al-Kubati et al. 200641 | • Cross-sectional design • Volunteers smoked for 45-minutes in a laboratory environment • Tobacco cessation prior to beginning of study: 12 hours • Czech Republic |
• Age range: 20 – 40 years old • Average age in years (Mean ± SD): 27 ± 6 • Average WP use in years (Mean ± SD): Not reported • n = 20 (100% male) • Convenience sampling |
• WP dimensions NR • 5 grams of Maassel shisha • SBP, DBP, and HR measured by photo-plethysmographic transducer (Finapres Ohmeda) |
• Small sample size • Only analyzed acute effects |
| Shaikh et al. 200842 | • Randomized cross-sectional • Trained pharmacy students interviewed WP users • Tobacco cessation prior to beginning of experiment: NR • Jordan |
• Age range: > 17 years old • Average age in years for sample (Mean ± SD): 31.4 ± 14.2 • Average WP use in years: Not reported • n = 14,310 (51.7% male) • Random sampling |
• WP dimensions not specified • Shisha amount or brand not specified • BP measured by mercury sphygmomanometer • MAP and HR were calculated |
• Self-reported smoking history • 78% of sample were students from Jordan |
| Blank et al. 20117 | • Double-blind design • Volunteers smoked for 45-minutes ad lib in a well ventilated laboratory setting • Tobacco cessation prior to start of experiment: NR • Richmond, VA |
• Age range: 18 – 50 years old • Average age in years (Mean ± SEM): 20.5 ± 2.1 • Average WP use every month (Mean ± SEM): 3.8 ± 1.0 • Average WP use ≥ 6 months (Mean ± SEM): 20.2 ± 12.9 • n = 37 (78% male) • Convenience sampling |
• WP dimensions:
o Chrome body: 43 cm and acrylic Base• 10 gram of shisha • BP and HR measured by Criticare Systems • CO measured by Vitalograph • COHb measured by venous blood sampling. NPT7 blood gas analyzer |
• Participants were casual users • Topography data varied from prior studies • Experiment conducted in lab setting • Smokers smoked alone; not in group setting |
| Hakim et al. 201110 | • Prospective study design • Volunteers smoked for 30-min ad lib on an open-air balcony • Tobacco cessation prior to start of experiment: at least 6 hours from cigarettes and at least 24 hours from WP smoking • Israel |
• Age range: 18.3 – 65.1 years old • Average age in years (Mean ± SD): 32.35 ± 23.36 • Average WP use in years: NR • n = 45 (66.67% male) • Convenience sampling |
• WP dimensions:
o 3.5 cm diameter; 1 cm width charcoal disk• 10 grams of double-apple flavored shisha from Nakhla • BP measured by Omron HEM-712 C BP digital • HR measurement technique NR • COHb measured by venous blood samples using an Illex cooximeter |
• Small sample size • Focused solely on health individuals • Did not analyze aldehydes, PAHs, and nicotine |
| Cobb et al. 201236 | • Cross-over study design • Volunteers smoked for 45-min with puff topography monitored in a laboratory setting • Tobacco cessation prior to start of experiment: 12 hours • Richmond, VA |
• Age range: 18 – 35 years old • Average age in years (Mean ± SD): 22.7 ± 4.8 • Average WP use in years (Mean ± SD): 2.2 ± 1.6 • n = 32 (50% male) • Convenience sampling |
• WP dimensions:
o Same as Blank et al. 20117• 10 gram of shisha • BP and HR measured by Model 507E, Criticare Systems inflatable cuff • CO measured by Vitalograph |
• Description of the demographics of the sample was unclear |
| Selim et al (a) 201343 | • Observational case-control study design • Tobacco cessation prior to start of experiment: Not reported • Cairo, Egypt |
• Age range: 25 – 35 years old • Average age in years of solely WP user (Mean ± SD): 28 ± 3 • Average WP use: 50% of WP smokers smoked > 5 years • Total n = 70 (90% male) • Solely WP smoker n = 30 (83.3% male) • Convenience sampling |
• WP dimensions NR • Shisha amount or brand NR • BP measurement technique NR |
• Brachial artery duplex ultrasonography needs to be evaluated as a screening tool for endothelial dysfunction |
| Selim et al (b) 201344 | • Prospective cohort study design • 1st time coronary angiography patients: May to October 2010 • Tobacco cessation prior to start of experiment: NR • Cairo, Egypt |
• Age range: Not specified • Average age in years (Mean ± SD): NR • Average WP use in years: Not specified • Total n = 287 (% male not reported) • Solely WP smoker n = 65 (% male NR • Convenience sampling |
• WP dimensions NR • Shisha amount or brand NR • BP measurement technique NR |
• Only one site • Self-reported smoking history • Most patients were on anti-ischemic treatment |
| Alomari et at 201445 | • Time-series design • Volunteers smoked ad lib for 30 minutes in a well-vented and air-conditioned room • Tobacco cessation prior to start of experiment: NR • Jordan |
• Age range: 18 – 35 years • Average age in years (Mean ± SD): 22.7 ± 4.8 • Average WP use in years: All participants smoked for >1 year • n = 53 (64% male) • Convenience sampling |
• WP dimensions
o Fast-lit charcoal (Three Kings, Holland)• 10 grams Two Red Apples, Nakhla, Egypt • BP measured by Omron HEM-907XL • HR measured by Auscultation • MAP calculated |
• Limited information on study demographic |
| Kadhum et al. 201446 | • Cross-sectional study design • Volunteers smoked ad lib for a period between 45 and 90 minutes in WP cafes • Tobacco cessation prior to start of experiment: NR • London, England |
• Age range: 18 – 25 years • Average age in years (Mean ± SD): NR • Average WP use in years: NR • n = 61 (80% male) • Convenience sampling |
• WP dimensions:
o Cafes used the same brand coal• Shisha weight NR • BP measured by manual sphygmomanometer • HR measured manually by palpation over 30 s • MAP measurement technique not reported • CO levels measured by portable CO monitor |
• WPS sessions varied (45 – 90 min) • Readings of BP, CO, MAP, and HR not consistent |
| Layoun et al. 201447 | • Observational non-invasive design • Smoked ad lib for a period between 60 and 90 min • Tobacco cessation prior to start of experiment: NR • Beirut and Mt Lebanon |
• Age range: NR • Average age in years for WP smokers (Mean ± SD): 29.26 ± 9.78 • Average WP use per week (Mean ± SD): 11.12 ± 17.27 • Total n = 132 (32% WP smokers) • Solely WP smokers n = 42 (78.6% male) • Convenience sampling |
• WP dimensions:
o Commercially available quick-lighting type charcoal• 20 grams of Muassel shisha • BP and HR measured by Beurer BM16 |
• Difficulty controlling puff topography and amount inhaled • No biomarkers of exposure |
| Shishani et al. 20148 | • Repeated-measures design • Smoked ad lib between 45 and 60 minutes in a fenced outdoor area until a defined target volume was achieved. • Tobacco cessation prior to start of experiment: 24 hours • Washington, USA |
• Age range: 18 – 30 years • Average age in years for WP smokers (Mean ± SD): 23 ± 3.1 • Average WP use: Smoked WP at least 10 times in past year but no more than twice a week in the 3 months before the experiment • n = 22 (59% male) • Convenience sampling |
• WP dimensions:
o 30 mm diameter; 5.6 grams; Golden Coal• 15 grams of Al Fakher shisha • BP and HR measured by Welch-Allyn monitor • CO measured by Bedford Micro + Smokerlyzer |
• Small sample size • Sessions took place in a laboratory setting |
| Azar et al. 201648 | • Trial design study • Smoked ad lib at 6 different restaurants • Tobacco cessation prior to start of experiment: 1 hour for cigarette and 12 hour WP • Lebanon |
• Age range: NR • Average age in years for WP smokers (Mean ± SD): 36 ± 13 • Average WP use: NR • Total n = 194 • WPS n = 101 (65% male) • Convenience sampling |
• WP dimensions: NR • Amount of shisha NR • BP and HR measured by automatic sphygmomanometer |
• Small number of controls • Type and dose of exposure varied for participants |
| Eissenberg et al. 200928 | • Two-condition crossover design • Volunteers smoked ad lib in a laboratory setting • Tobacco cessation prior to beginning of experiment: 12 hours • Richmond, VA |
• Age range: 18 – 50 years • Average age in years (Mean ± SD): 21.4 ± 2.3 • Average WP use per month (Mean ± SD): 5.2 ± 4.0 • WPS n = 31 (67.7% male) • Convenience sampling |
• WP dimensions:
o Same as Blank et al. 20117• 15 grams of shisha • HR measured by Model 506, Criticare Systems, fitted with a reusable finger pulse oximeter sensor • CO measured by Vitalograph • COHb measured by NPT7 blood gas analyzer |
• Smoking took place in a laboratory setting • WP smokers also smoked cigarettes |
Abbreviations: BP, blood pressure; bpm, beats per minute; CO, carbon monoxide; COHb, carboxyl hemoglobin; DBP, diastolic blood pressure; HR, heart rate; MAP, mean arterial pressure; mmHG, millimeter of mercury; n, sample size; NR, not reported; s, seconds; SBP, systolic blood pressure; WP, waterpipe; WPS, waterpipe smoke; PAH, polyaromatic hydrocarbons; UAE, United Arab Emirates
Fifteen studies were used in the meta-analyses to determine the effects of WPS on CVD and respiratory markers of tobacco exposure by assessing changes in intermediate hemodynamic parameters affecting cardiopulmonary function, including MAP, SBP, DBP, and HR, as well as expired CO and COHb concentration to assess WPS exposure18. A pooled analysis of the results revealed an association between a WPS session and an increase in mean SBP and DBP of 6.45 mmHg (95% CI 3.87 to 9.04; p < 0.0001) and 3.71 mmHg (95% CI 2.34 to 5.08; p < 0.0001), respectively (Figure 2). Additionally, MAP increased by 5.54 mmHg (95% CI 3.33 to 7.76; p < 0.0001), following a WPS session (Figure 3). There was also an increase in COHb levels by 4.11% (95% CI 3.38 to 4.84; p < 0.0001) and expired CO by 22.53 ppm (95% CI 15.99 to 29.08; p < 0.0001) (Figures 4 and 5, respectively). Figure 6 illustrates that a WPS session is also associated with elevated HR by 7.03 bpm (95% CI 4.60 to 9.46; p < 0.0001). All the meta-analyses had substantial heterogeneity (>90%), which we accounted for by pooling the results with a random effects model.
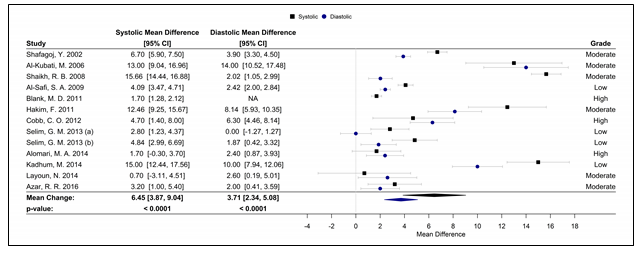
Figure 2. Forest plot of the mean difference of a WPS session and an acute elevation in SBP/DBP.
Abbreviations: CI, confidence interval, DBP, diastolic blood pressure, SBP, systolic blood pressure; WPS, waterpipe smoke
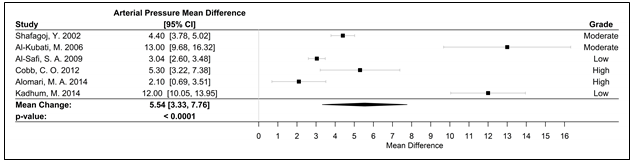
Figure 3. Forest plot of the mean difference of a WPS session and an acute elevation in MAP measured in mmHg.
Abbreviations: CI, confidence interval, MAP, mean arterial pressure; mmHg, millimeters of mercury; WPS, waterpipe smoke
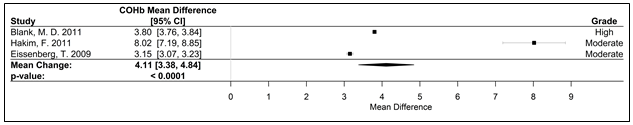
Figure 4. Forest plot of the mean difference of acute elevation in COHb measured as a percent.
Abbreviations: CI, confidence interval; COHb carboxyhemoglobin; WPS, waterpipe smoke
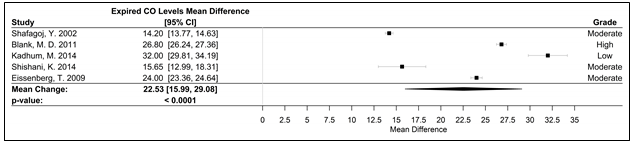
Figure 5. Forest plot of the mean difference of acute elevation in expired CO measured in ppm.
Abbreviations: CI, confidence interval, CO, carbon monoxide; ppm, parts per million; WPS, waterpipe smoke
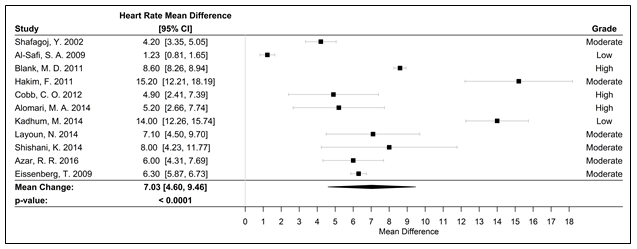
Figure 6. Forest plot of the mean difference of a WPS session and an acute elevation in HR measured in bpm
Abbreviations: bpm, beats per minute; HR, heart rate; WPS, water pipe smoke; CI, confidence interval
Using GRADE, the quality of evidence for each study was assessed. Three studies received a high GRADE, 8 were moderate GRADE, and 4 were low GRADE. To further analyze our data, we conducted a sensitivity analyses by stratifying by the GRADE of the studies and by type of WPS. Upon performing a subgroup analysis of only high/moderate GRADE studies, heterogeneity (I2 statistic) decreased from >90% to 0.0% in the meta-analysis for the outcome of SBP and 62.3% for DBP. Unfortunately, there was limited overlap in the type and size of the WPS between each study and many studies did not report this level of detail, thereby limiting our ability to conduct sensitivity analyses on these factors.
Discussion
WPS is a common practice with increasing prevalence globally, especially amongst younger generations19-21.. The perception that WPS carries fewer health risks than cigarette smoking has propelled its growth and contributed to limited regulations,19-21 despite the fact that several studies have shown that WPS may have similar health consequences as cigarette smoking20-24. The findings from our pooled analyses, showing statistically significant increases in all measured hemodynamic parameters of cardiopulmonary function, parallel the effects seen with smoking cigarettes25.
The nicotine in cigarettes has been found to increase systolic blood pressure 5-10 mmHg and HR by 10-15 bpm;25 similarly, our analysis found an increase in SBP by 6.45 mmHg and HR by 7.03 bpm. More recently, a Mendelian Randomization Meta-Analysis looking at the effect of smoking on blood pressure and resting heart rate measurements revealed that smoking one additional cigarette per day was associated with a 0.08 mmHg increase in SBP, 0.05 mmHg increase in DBP, and 0.21 bpm increase in HR26. Additionally, not only does the literature suggest comparable changes in hemodynamic parameters with acute use of cigarettes or WPS, but also that WPS may actually be associated with higher levels of exposure to some toxicants such as tobacco-specific nitrosamines, polycyclic aromatic hydrocarbons, volatile aldehydes, benzene, nitric oxide, heavy metals, and CO due to the burning of charcoal to heat tobacco- or nontobacco-based shisha in the hookah WP20,21,27,28. In a direct comparison of toxicant exposure between WPS and cigarette smoking, Eissenberg and Shihadeh in 2009 found that WPS was associated with an average increase in expired CO by 23.9 ppm whereas the average expired CO for cigarette smoking was 2.7 ppm28. However, the peak nicotine levels for WPS and tobacco cigarettes were similar in the study,28 which can explain why WPS has similar effects on hemodynamic parameters as does smoking cigarettes. In fact, the level of nicotine in the blood can increase up to 250% after one session of hookah smoking and remain elevated for 40-45 minutes20,21. Of course, the dose of nicotine and other harmful and potentially harmful constituents varies with the duration and intensity of both hookah and smoking cigarettes.
As with smoking cigarettes, the changes seen in hemodynamic parameters of cardiopulmonary function in this meta-analysis can be attributed to the nicotine and CO exposures obtained with WPS20,21,25,30. Nicotine is an agonist at nicotinic receptors in the brain, at neuromuscular junctions, the adrenal medulla, and autonomic ganglia. It acutely increases norepinephrine and epinephrine release, which in turn increases heart rate, myocardial contractility, and cardiac output20,25,30,31. High levels of CO can lead to decreased oxygen supply to tissues, including myocardial muscles, due to the production of COHb, which prevents the binding and distribution of oxygen to the body20,25,32. Lastly, exposure to additional toxicants in WPS can lead to oxidative stress, which in turn can induce inflammation and endothelial dysfunction25,33. Together, these factors combine to increase myocardial oxygen demand and decrease in oxygen supply, putting strain on the heart, and can also lead to vascular damage20,25,30,31. Although acutely these changes lead to increases in blood pressure and HR, cumulative exposures are beginning to show a trend toward development of cardiopulmonary disease20,24.
This meta-analysis indicates that the acute effect of WPS on the cardiopulmonary system parallels smoking cigarettes and long term studies are also starting to show a trend toward negative health effects34. Similarly, Haddad, et al.29 performed a systematic review and found that WPS may be as harmful as cigarettes in several hemodynamic parameters of cardiopulmonary function, but regulations of WPS remain limited21. In the United States, hookah establishments are often exempted from smoke free air laws in several states,21 but some hookah establishments must use tobacco-free shisha instead of the traditional tobacco shisha35. Importantly, even tobacco-free shisha can affect hemodynamic parameters. Despite the absence of nicotine, tobacco-free shisha can acutely increase expired CO and COHb levels and has been shown to compromise cardiopulmonary regulation7,36. As the evidence on the negative cardiopulmonary, and overall, health effects of WPS grows, policy changes will be necessary to protect the public37.
In this meta-analysis, we found statistically significant increases in hemodynamic parameters of cardiopulmonary function and expired CO content after WPS exposure, but a limitation was the high heterogeneity of the data. This can be attributed to the geographic diversity of the studies analyzed, the multiple study protocols, and the various different forms of waterpipes, shisha, and charcoal that exist, as noted by Chaouachi et al38-40 as a serious limitation of the existing WPS literature. For future studies, standardized approaches to evaluating WPS exposures and outcomes across a varied global population will be necessary to more definitively associate WPS with long term cardiopulmonary disease.
Conclusion
WPS exposure is associated with statistically significant and potentially clinically relevant short term increases in hemodynamic parameters of cardiopulmonary function, including SBP, DBP, MAP, HR, and the amount of expired CO. Importantly, these findings parallel the acute effects seen with smoking cigarettes. Studies looking at the long-term effects of repeated WPS exposures on cardiopulmonary disease are in their infancy, but initial results point towards development of cardiopulmonary disease with cumulative WPS exposure. If the trend toward cardiopulmonary disease persists, health policy changes will need to take place in response to the negative cardiopulmonary health effects of WPS. However, standardized approaches to evaluating WPS exposures and outcomes are necessary for more definitive associations with health outcomes.
Acknowledgements
Ashley Forrest Curran, MLS, at NYU Health Sciences Library, New York, New York
Funding
For this publication, the American Heart Association Tobacco Regulation and Addiction Center supported the research (Grant 1P50HL120163-01).
Conflict of Interest
Mr. Matthew Marshall, Ms. Marya Ghazipura, and Drs. Tanzib Hossain, Terry Gordon, and Lung-Chi Chen have no conflicts of interest to disclose.
References
- Lozano R. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012; 380: 2095-2128. doi:10.1016/S0140-6736(12)61728-0.
- National Center for Health Statistics. Health, United States, 2016: With Chartbook on Long-term Trends in Health. Hyattsville, MD. 2017. https://www.cdc.gov/nchs/data/hus/hus16.pdf
- Mozaffarian D. Executive summary: Heart Disease and Stroke Statistics-2016 update: A report from the American Heart Association. Circulation. 2016; 133: 447.
- American Cancer Society. Cancer Facts & Figures 2017. Atlanta: American Cancer Society; 2017.
- Health UDo, Services H. The 2004 United States surgeon general’s report: the health consequences of smoking. NSW Public Health Bull. 2004; 15: 107.
- National Center for Health Statistics. Health, United States, 2014: With Special Feature on Adults Aged 55–64. Hyattsville, MD. 2015. https://www.cdc.gov/nchs/data/hus/hus14.pdf
- Blank MD. Acute effects of waterpipe tobacco smoking: a double-blind, placebo-control study. Drug Alcohol Depend. 2011; 116: 102-109. doi:10.1016/j.drugalcdep.2010.11.026.
- Shishani K, Howell D, McPherson S, et al. Young adult waterpipe smokers: Smoking behaviors and associated subjective and physiological effects. Addictive behaviors. 2014; 39: 1113-1119. doi:10.1016/j.addbeh.2014.03.010.
- Amrock SM, Gordon T, Zelikoff JT, et al. Hookah use among adolescents in the United States: results of a national survey. nicotine & tobacco research ntt160. 2013.
- Hakim F. The acute effects of water-pipe smoking on the cardiorespiratory system. Chest. 2011; 139: 775-781. doi:10.1378/chest.10-1833.
- Waziry R, Jawad M, Ballout RA, et al. The effects of waterpipe tobacco smoking on health outcomes: an updated systematic review and meta-analysis. International journal of epidemiology, dyw021. 2016.
- Shafagoj YA, Mohammed FI. Levels of maximum end-expiratory carbon monoxide and certain cardiovascular parameters following hubble-bubble smoking. Saudi medical journal. 2002; 23: 953-958.
- Al-Safi SA, Ayoub NM, Mosa’b AA, et al. Does shisha smoking affect blood pressure and heart rate. Journal of Public Health. 2009; 17: 121-126.
- Moher DLA, Tetzlaff J, Altman DG. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Medicine. 6: e10000097.
- Guyatt GH OA, Schunemann HJ, Tugwell P, et al. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 64.
- Schwarzer G. meta: An R package for meta-analysis. R News. 2007; 7: 40-45.
- Gordon ML, Thomas. Forestplot: Advanced Forest Plot Using 'grid' Graphics. R package version 1.7.
- Cohn JN, Quyyumi AA, Hollenberg NK, et al. Surrogate markers for cardiovascular disease: functional markers. Circulation. 2004; 109: IV31-46, doi:10.1161/01.CIR.0000133442.99186.39.
- Knishkowy B, Amitai Y. Water-pipe (narghile) smoking: an emerging health risk behavior. Pediatrics. 2005; 116: e113-119, doi:10.1542/peds.2004-2173.
- WHO Study Group on Tobacco Product Regulation. “Waterpipe tobacco smoking: health effects, research needs, and recommended actions for regulators – 2nd Edition.” 2015. http://apps.who.int/iris/bitstream/10665/161991/1/9789241508469_eng.pdf?ua=1&ua=1.
- American Lung Association. “An Emerging Deadly Trend: Waterpipe Tobacco Use.” American Lung Association Tobacco Policy Trend Alert. February 2007. http://www.lungusa2.org/embargo/slati/Trendalert_Waterpipes.pdf.
- Raad D. Effects of water-pipe smoking on lung function: a systematic review and meta-analysis. Chest. 2011; 139: 764-774, doi:10.1378/chest.10-0991.
- Chan A, Murin S. Up in smoke: the fallacy of the harmless Hookah. Chest. 2011; 139: 737-738, doi:10.1378/chest.10-2985.
- Sibai AM. Lifetime cumulative exposure to waterpipe smoking is associated with coronary artery disease. Atherosclerosis. 2014; 234: 454-460, doi:10.1016/j.atherosclerosis.2014.03.036.
- Benowitz NL. Cigarette smoking and cardiovascular disease: pathophysiology and implications for treatment. Prog Cardiovasc Dis. 2003; 46: 91-111.
- Linneberg A. Effect of Smoking on Blood Pressure and Resting Heart Rate: A Mendelian Randomization Meta-Analysis in the CARTA Consortium. Circulation. Cardiovascular genetics. 2015; 8: 832-841, doi:10.1161/circgenetics.115.001225.
- Juhasz. Kinetics of exhaled carbon monoxide following waterpipe smoking indoors and outdoors. Chest. 2017.doi:10.1016/j.chest.2017.02.006.
- Eissenberg T, Shihadeh A. Waterpipe tobacco and cigarette smoking: direct comparison of toxicant exposure. Am J Prev Med. 2009; 37: 518-523, doi:10.1016/j.amepre.2009.07.014.
- Haddad L. A Systematic Review of Effects of Waterpipe Smoking on Cardiovascular and Respiratory Health Outcomes. Tobacco use insights. 2016; 9: 13-28, doi:10.4137/tui.s39873.
- U.S. Department of Health and Human Services. “The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General.” Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. https://www.surgeongeneral.gov/library/reports/50-years-of-progress/full-report.pdf.
- Salahuddin S, Prabhakaran D, Roy A. Pathophysiological Mechanisms of Tobacco-Related CVD. Global heart. 2012; 7: 113-120, doi:10.1016/j.gheart.2012.05.003.
- Weaver LK. Carbon monoxide poisoning. New England Journal of Medicine. 2009; 360: 1217-1225.
- Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004; 43: 1731-1737. doi:10.1016/j.jacc.2003.12.047.
- Akl EA. The effects of waterpipe tobacco smoking on health outcomes: a systematic review. International journal of epidemiology. 2010; 39: 834-857. doi:10.1093/ije/dyq002.
- Zhou S. Air quality in New York City hookah bars. Tobacco control, tobaccocontrol-2014-051763 2014.
- Cobb CO, Sahmarani K, Eissenberg T, et al. Acute toxicant exposure and cardiac autonomic dysfunction from smoking a single narghile waterpipe with tobacco and with a "healthy" tobacco-free alternative. Toxicol Lett. 2012; 215: 70-75. doi:10.1016/j.toxlet.2012.09.026.
- Noonan D, Kulbok PA. New tobacco trends: waterpipe (hookah) smoking and implications for healthcare providers. Journal of the American Academy of Nurse Practitioners. 2009; 21: 258-260, doi:10.1111/j.1745-7599.2009.00402.x.
- Chaouachi K. Assessment of narghile (shisha, hookah) smokers' actual exposure to toxic chemicals requires further sound studies. The Libyan journal of medicine. 2011; 6. doi:10.3402/ljm.v6i0.5934.
- Chaouachi K. More rigor needed in systematic reviews on "waterpipe" (hookah, narghile, shisha) smoking. Chest. 2011; 139: 1250-1251. author reply 1251-1252, doi:10.1378/chest.10-2864.
- Chaouachi K. Hookah (shisha, narghile, "water pipe") indoor air contamination in German unrealistic experiment. Serious methodological biases and ethical concern. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2010; 48: 992-995. author reply 996-997, doi:10.1016/j.fct.2010.01.020.
- Al-Kubati M, Al-Kubati AS, al'Absi M, et al. The short-term effect of water-pipe smoking on the baroreflex control of heart rate in normotensives. Autonomic neuroscience : basic & clinical. 2006; 126-127: 146-149. doi:10.1016/j.autneu.2006.03.007.
- Shaikh RB, Vijayaraghavan N, Sulaiman AS, et al. The acute effects of Waterpipe smoking on the cardiovascular and respiratory systems. Journal of preventive medicine and hygiene. 2008; 49: 101-107.
- Selim GM, Elia RZ, El Bohey AS, et al. Effect of shisha vs. cigarette smoking on endothelial function by brachial artery duplex ultrasonography: an observational study. Anadolu kardiyoloji dergisi : AKD = the Anatolian journal of cardiology. 2013; 13: 759-765. doi:10.5152/akd.2013.4499.
- Selim GM, Fouad H, Ezzat S. Impact of shisha smoking on the extent of coronary artery disease in patients referred for coronary angiography. Anadolu kardiyoloji dergisi : AKD = the Anatolian journal of cardiology. 2013; 13: 647-654. doi:10.5152/akd.2013.191.
- Alomari MA, Khabour OF, Alzoubi KH, et al. Central and peripheral cardiovascular changes immediately after waterpipe smoking. Inhalation toxicology. 2014; 26: 579-587. doi:10.3109/08958378.2014.936572.
- Kadhum M, Jaffery A, Haq A, et al. Measuring the acute cardiovascular effects of shisha smoking: a cross-sectional study. JRSM open. 2014; 5: 2054270414531127, doi:10.1177/2054270414531127.
- Layoun N. Waterpipe effects on pulmonary function and cardiovascular indices: a comparison to cigarette smoking in real life situation. Inhalation toxicology. 2014; 26: 620-627. doi:10.3109/08958378.2014.945106.
- Azar RR. Acute effects of waterpipe smoking on blood pressure and heart rate: a real-life trial. Inhalation toxicology. 2016; 28: 339-342, doi:10.3109/08958378.2016.1171934.
